Pelargonium sidoides also known as Umckaloabo, is a herbal remedy thought to be effective in the therapy of acute respiratory infections. EPs 7630 is a specific extract from the roots of the P. sidoides. To examine the efficacy of therapy with EPs 7630 in patients with acute bronchitis, a multi-centre, prospective, open observational study was performed in 440 study sites located in Germany. Clinical studies have shown that EPs 7630, an aqueous ethanolic extract from the roots of umckaloabo, is an effective therapy for respiratory tract infections. (EPs 7630 is a registered trademark of Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany).
Medicinal Effects
In vitro studies demonstrate that Pelargonium sidoides has antiviral, antibacterial, immunomodulatory effects. In 1972, German scientists discovered the chemical profile identity of the Pelargonium sidoides root. As research progressed, a specific extraction technique was developed and perfected to efficiency EPs 7630. It was determined that 3-year-old rhizomes contain an optimum amount of active constituents. EPs 7630 has been shown to be safe and effective in the therapy of acute upper respiratory tract infections, including bronchitis, sinusitis, tonsillopharyngitis, and common cold. Medical researches have suggested that the mechanism of action of EPs 7630 is multifactorial including antiviral and antibacterial effects and noteworthy immune modulatory capabilities as well as cytoprotective activities. The efficacy of EPs 7630 in the therapy of acute respiratory tract infections (ARTI) has been evaluated in over 3500 patients of placebo-controlled double-blind studies and in over 5500 patients of open-label and non-interventional studies. Out of the total of over 9000 participants, approximately 4000 were children and adolescents.
In a 2003 study from “Alternative Therapies in Health and Medicine“, enrolled 143 children aged 6-10 years with a nondangerous form of strep throat. On average, the total duration of the illness was reduced by 2 days in the Pelargonium group as compared to the placebo group. Randomized controlled a clinical trial 103 patients with nasal congestion, cough, sore throat, hoarseness, sneezing, scratchy throat, headache, muscle aches, and fever were evaluated. After 10 days of therapy 78 percent of treated patients 32 percent of the placebo group had complete relaxation of symptoms. In Feb 2007, a study was reported that tested pelargonium sidoides in 217 participants with acute bronchitis. Subjects were given either placebo or P. sidoides extract at a dose of 30 drops daily. After 7 days of therapy, participants given the Pelargonium treatment demonstrated significantly better scores than those given placebo. Particularly, improvements were seen in cough, mucus, wheezing, and shortness of breath.
In a study 124 adults aged 18 years and over with a BSS (Bronchitis Severity Score) of greater than 5 points were randomized to Umckaloabo liquid or placebo. Participants who were administered Umckaloabo had a greater decreased in Bronchitis Severity Score than those on placebo. Scientists at the “University Hospital Freiburg” analyzed 406 patients throughout 7 day-long clinical trial. Three of the four groups received different doses of EPs 7630, 3 times daily. The last group received a placebo 3 times daily. The experts used a scoring method to evaluate the overall change in bronchitis symptoms and noted that all 3 EPs 7630 therapy groups saw a noteworthy reduce in symptoms compared to the placebo group. In a clinical study with 468 participant with bronchitis received 4.5 ml of the P. sidoides extract or a placebo for seven days. Important differences were found between the 2 group, with the umckaloabo extracts group reporting less severity of bronchitis symptoms. At the start of the study, 67 percent of the patients were unable to work. Just 16 percent of the umcka extract group was unable to work while 43 percent of the placebo group was still unable to work.
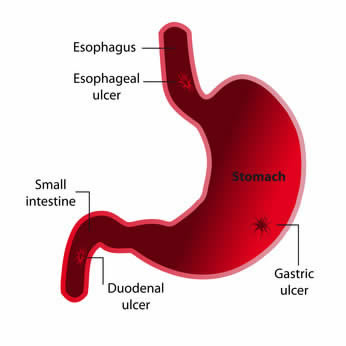 Different studies in animals and humans have showed favorable effects from the use of DGL (Deglycyrrhizinated licorice) in gastric and duodenal ulcer conditions. DGL (Deglycyrrhizinated licorice) has been shown to reduce gastric bleeding caused by aspirin, therefore, it is strongly indicated for prevention of ulcers in patients requiring long term cure with ulcerogenic medications. One animal-based study found that aspirin coated with licorice root reduced the number of ulcers in rats by 50%. A human study showed that fecal blood loss induced by aspirin (375 mg 3 times daily) was significantly reduced with coadministration of deglycyrrhizinated licorice (175 mg/dose aspirin). Some studies have showed that DGL is as effective as ranitidine and cimetidine for both therapy and maintenance treatment of gastric ulcers. In a study involving 100 gastric ulcer patients, half received the acid-blocking medication
Different studies in animals and humans have showed favorable effects from the use of DGL (Deglycyrrhizinated licorice) in gastric and duodenal ulcer conditions. DGL (Deglycyrrhizinated licorice) has been shown to reduce gastric bleeding caused by aspirin, therefore, it is strongly indicated for prevention of ulcers in patients requiring long term cure with ulcerogenic medications. One animal-based study found that aspirin coated with licorice root reduced the number of ulcers in rats by 50%. A human study showed that fecal blood loss induced by aspirin (375 mg 3 times daily) was significantly reduced with coadministration of deglycyrrhizinated licorice (175 mg/dose aspirin). Some studies have showed that DGL is as effective as ranitidine and cimetidine for both therapy and maintenance treatment of gastric ulcers. In a study involving 100 gastric ulcer patients, half received the acid-blocking medication 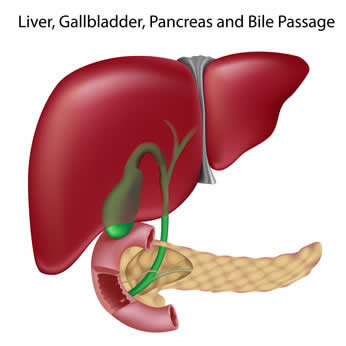 In a study conducted in Iran, the scientists treated 55 patients with active and chronic cases of hepatitis C infections with 630 mg a day of silymarin for 24 weeks. After 24 weeks of therapy, the ALT liver enzyme levels decreased down from an average of 108 U/L to an average of 70, and AST levels decreased from an average of 99 U/L to an average of 60. Quality of life scores improved substantially and of the 55 patients treated, 9 had HCV-negative RNA levels after the therapy. In a 6-month double-blind study of 36 patients with chronic alcoholic liver illness, the group given silymarin (Legalon) showed normalization of their bilirubin, aspartate transaminase and alanine transaminase serum levels. In different study, 106 patients with mild acute and subacute liver illness characterized by elevated serum transaminase levels were randomized to receive silymarin or placebo. Of the 97 patients who completed the 4-week study, there was a statistically considerable greater decrease in transaminase levels in the silymarin group.
In a study conducted in Iran, the scientists treated 55 patients with active and chronic cases of hepatitis C infections with 630 mg a day of silymarin for 24 weeks. After 24 weeks of therapy, the ALT liver enzyme levels decreased down from an average of 108 U/L to an average of 70, and AST levels decreased from an average of 99 U/L to an average of 60. Quality of life scores improved substantially and of the 55 patients treated, 9 had HCV-negative RNA levels after the therapy. In a 6-month double-blind study of 36 patients with chronic alcoholic liver illness, the group given silymarin (Legalon) showed normalization of their bilirubin, aspartate transaminase and alanine transaminase serum levels. In different study, 106 patients with mild acute and subacute liver illness characterized by elevated serum transaminase levels were randomized to receive silymarin or placebo. Of the 97 patients who completed the 4-week study, there was a statistically considerable greater decrease in transaminase levels in the silymarin group.