Ubiquinol may be important for patients taking cholesterol-lowering statin medications because statin medications can reduce Coenzyme Q10 levels in the body. It has a protective property on red blood cells, helping especially to maintain levels during long-term therapy with statins.
Statin Drugs and CoQ10 Deficiency
Naturally produced in bodies, ubiquinol is an active form of CoQ10 (Coenzyme Q10), which has been shown to have quite strong antioxidant property. As an antioxidant, Coenzyme Q10 helps protect proteins and mitochondrial DNA from oxidative damage and, supports healthy heart. The level of CoQ10 in tissues decreases as people get older. Some data state that this decline in Coenzyme Q10 begins around age 20, and noticeable declines in Coenzyme Q10 in the heart and other organs becomes apparent after the age of 40. In 1977 the “Journal of Orthomolecular Medicine” reported that a 77 year old person has 57 % less Coenzyme Q10 than a 20 year old. An analysis of heart muscle tissue collected from people with heart disease showed a marked decrease in the tissue Coenzyme Q10 concentration. It has been shown that patients with lower ubiquinol concentrations and decreases in ATP production in the heart muscle tissue suffered more serious types of heart disease than individuals with higher levels of Coenzyme Q10.
HMA Co-A reductase inhibitors or statin drugs inhibit one of the important steps in CoQ10 synthesis. These medications have been associated with a diminution in serum and muscle tissue coenzyme Coenzyme Q10 levels and may play a role in statin-induced myopathy. Statin treatment reduces blood Coenzyme Q10 concentrations. Statin-induced Coenzyme Q10 depletion is well documented in animal and human studies with deleterious cardiac consequences in both animal models and human studies. Because of this close correlation, co-administration of CoQ10 along with statins is recommended by some doctors to prevent or treat the adverse effects of statin drugs.
A Columbia University study found that 30 days of statin treatment (80 mg/day) decreased Coenzyme Q10 levels by half. In 2004, a study reported in the “American Journal of Cardiology” documented evidence of early heart muscle weakness in 70% of patients taking statin medication treatment for a period of 6 months. Ubiquinol may help limit muscle pain from taking statins and help avoid rhabdomyolysis. A double-blind study found CoQ10 can decrease muscle pain associated with statin medication use. Participants were given 100 mg Coenzyme Q10 for 30 days finding pain severity decreased by 40 percent, and pain interference with daily activities decreased by 38 percent in the Coenzyme Q10 group.
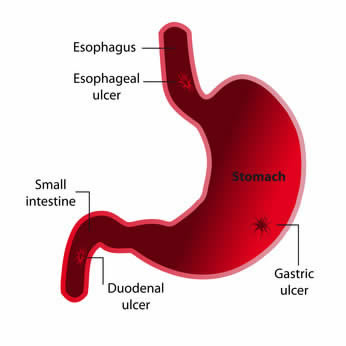 Different studies in animals and humans have showed favorable effects from the use of DGL (Deglycyrrhizinated licorice) in gastric and duodenal ulcer conditions. DGL (Deglycyrrhizinated licorice) has been shown to reduce gastric bleeding caused by aspirin, therefore, it is strongly indicated for prevention of ulcers in patients requiring long term cure with ulcerogenic medications. One animal-based study found that aspirin coated with licorice root reduced the number of ulcers in rats by 50%. A human study showed that fecal blood loss induced by aspirin (375 mg 3 times daily) was significantly reduced with coadministration of deglycyrrhizinated licorice (175 mg/dose aspirin). Some studies have showed that DGL is as effective as ranitidine and cimetidine for both therapy and maintenance treatment of gastric ulcers. In a study involving 100 gastric ulcer patients, half received the acid-blocking medication
Different studies in animals and humans have showed favorable effects from the use of DGL (Deglycyrrhizinated licorice) in gastric and duodenal ulcer conditions. DGL (Deglycyrrhizinated licorice) has been shown to reduce gastric bleeding caused by aspirin, therefore, it is strongly indicated for prevention of ulcers in patients requiring long term cure with ulcerogenic medications. One animal-based study found that aspirin coated with licorice root reduced the number of ulcers in rats by 50%. A human study showed that fecal blood loss induced by aspirin (375 mg 3 times daily) was significantly reduced with coadministration of deglycyrrhizinated licorice (175 mg/dose aspirin). Some studies have showed that DGL is as effective as ranitidine and cimetidine for both therapy and maintenance treatment of gastric ulcers. In a study involving 100 gastric ulcer patients, half received the acid-blocking medication  In his researches, Dr. Nieper proved that serratiopeptidase was capability of dissolving and digesting the substances that cause plaque formation on the arterial walls, such as cholesterol, calcium, cellular wastes, various fats, and fibrin, a clotting agent. Excessive plaque results in partial or complete blockage of blood flow through an artery, resulting in arteriosclerosis which could cause a heart attack or stroke. Dr. Nieper explained that serratiopeptidase digests non-living tissue, blood clots, cysts, arterial plaque and inflammation in all forms. It protects against strokes and has been found to be more effectual and fast than EDTA chelation therapies in removing arterial plaque. Serrapeptase acts to safely and effectively reverse chronic inflammation. In recent years, a growing number of sresearchers have come to realize that one of the primary causes for the deposits of deleterious, plaque-forming substances inside arterial walls is chronic, low-grade inflammation.
In his researches, Dr. Nieper proved that serratiopeptidase was capability of dissolving and digesting the substances that cause plaque formation on the arterial walls, such as cholesterol, calcium, cellular wastes, various fats, and fibrin, a clotting agent. Excessive plaque results in partial or complete blockage of blood flow through an artery, resulting in arteriosclerosis which could cause a heart attack or stroke. Dr. Nieper explained that serratiopeptidase digests non-living tissue, blood clots, cysts, arterial plaque and inflammation in all forms. It protects against strokes and has been found to be more effectual and fast than EDTA chelation therapies in removing arterial plaque. Serrapeptase acts to safely and effectively reverse chronic inflammation. In recent years, a growing number of sresearchers have come to realize that one of the primary causes for the deposits of deleterious, plaque-forming substances inside arterial walls is chronic, low-grade inflammation.